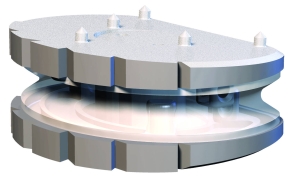
Cervical prosthesis CP-ESP
CP-ESP helps to reduce pain and to achieve near-normal disc function.
The stiffness of the deformable core resembles that of the native intervertebral discs. This stiffness is due to a flexible polycarbonate urethane cushion. This flexible cushion, attached to the two metal endplates, provides the disc with its shock-absorbing function and limits the release of particles into the body and the calcification of deformable parts.
The CP-ESP device is indicated for use in skeletally mature patients for reconstruction of the disc at one level from C3-C7 following single-level discectomy for intractable radiculopathy (arm pain and/or a neurological deficit) with or without neck pain, or myelopathy due to a single-level abnormality localized to the level of the disc space and manifested by at least one of the following conditions confirmed by radiographic imaging (e.g., X-rays, computed tomography (CT), magnetic resonance imaging (MRI)): herniated nucleus pulposus, spondylosis (defined by the presence of osteophytes), and/or visible loss of disc height as compared to adjacent levels. Patients receiving the CP-ESP device should have failed at least six weeks of non-operative treatment or have the presence of progressive symptoms (e.g., numbness or tingling) prior to implantation. The CP-ESP device is implanted via an open anterior approach.
CP-ESP is CE marked and approved for use in Europe. It is not approved by FDA for use in the US.