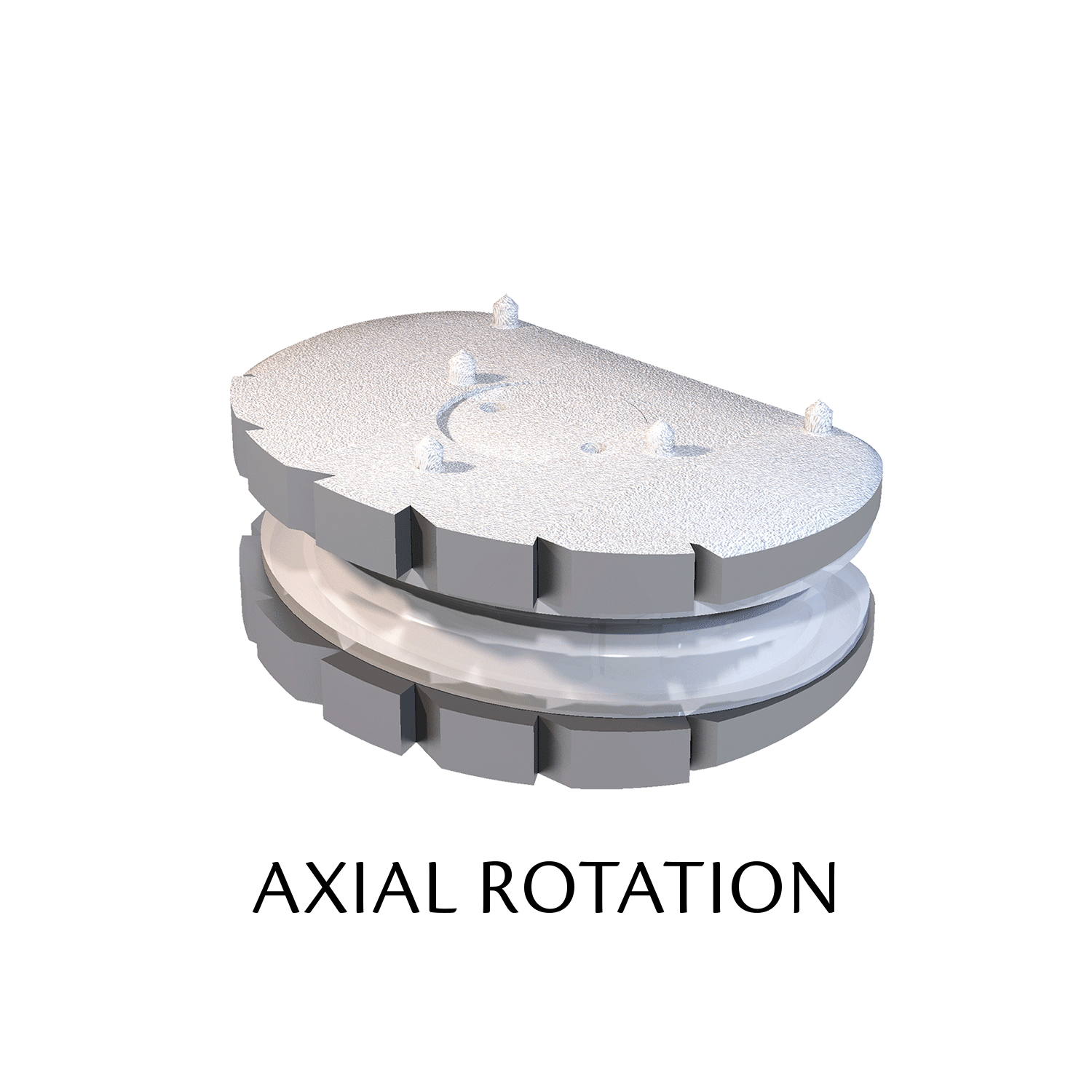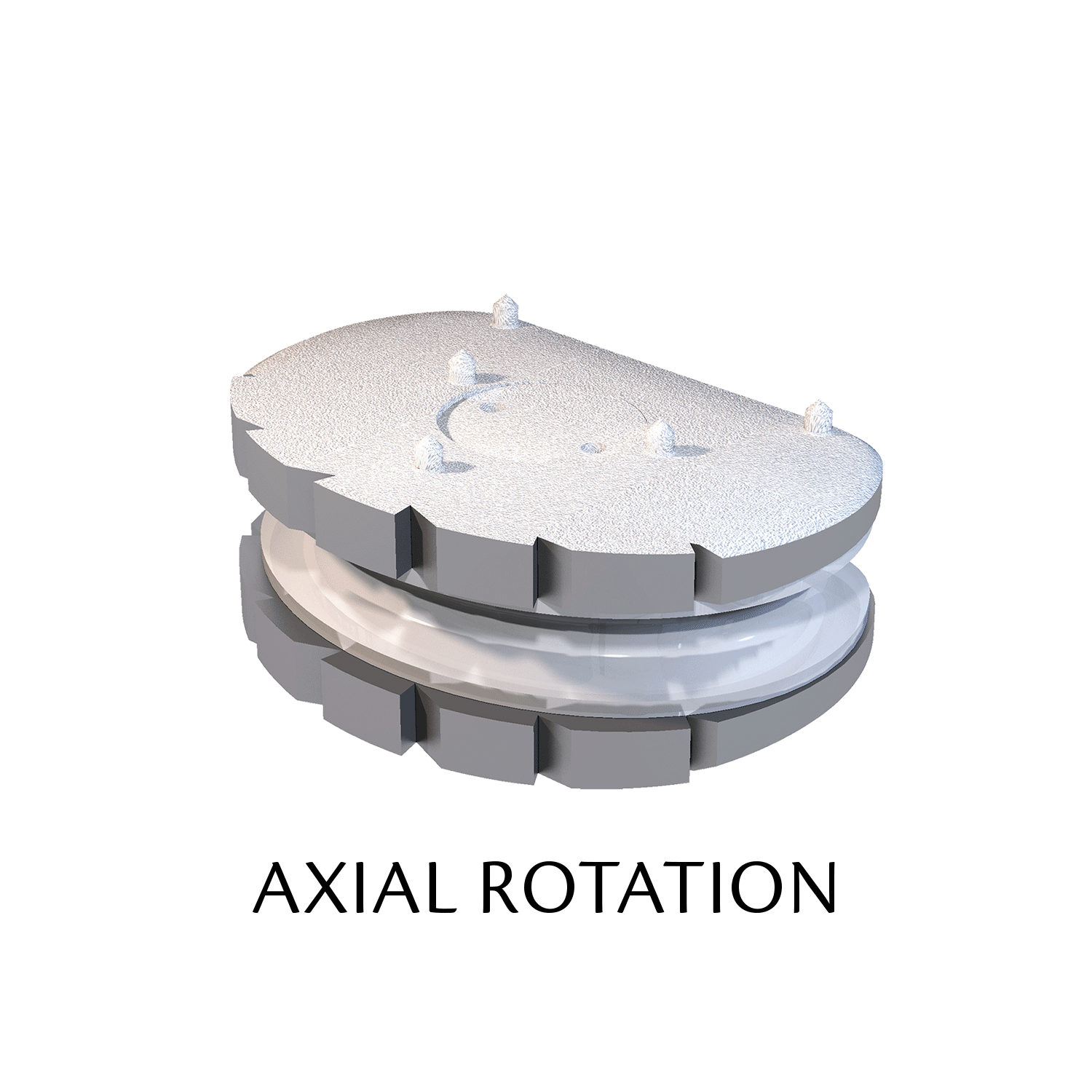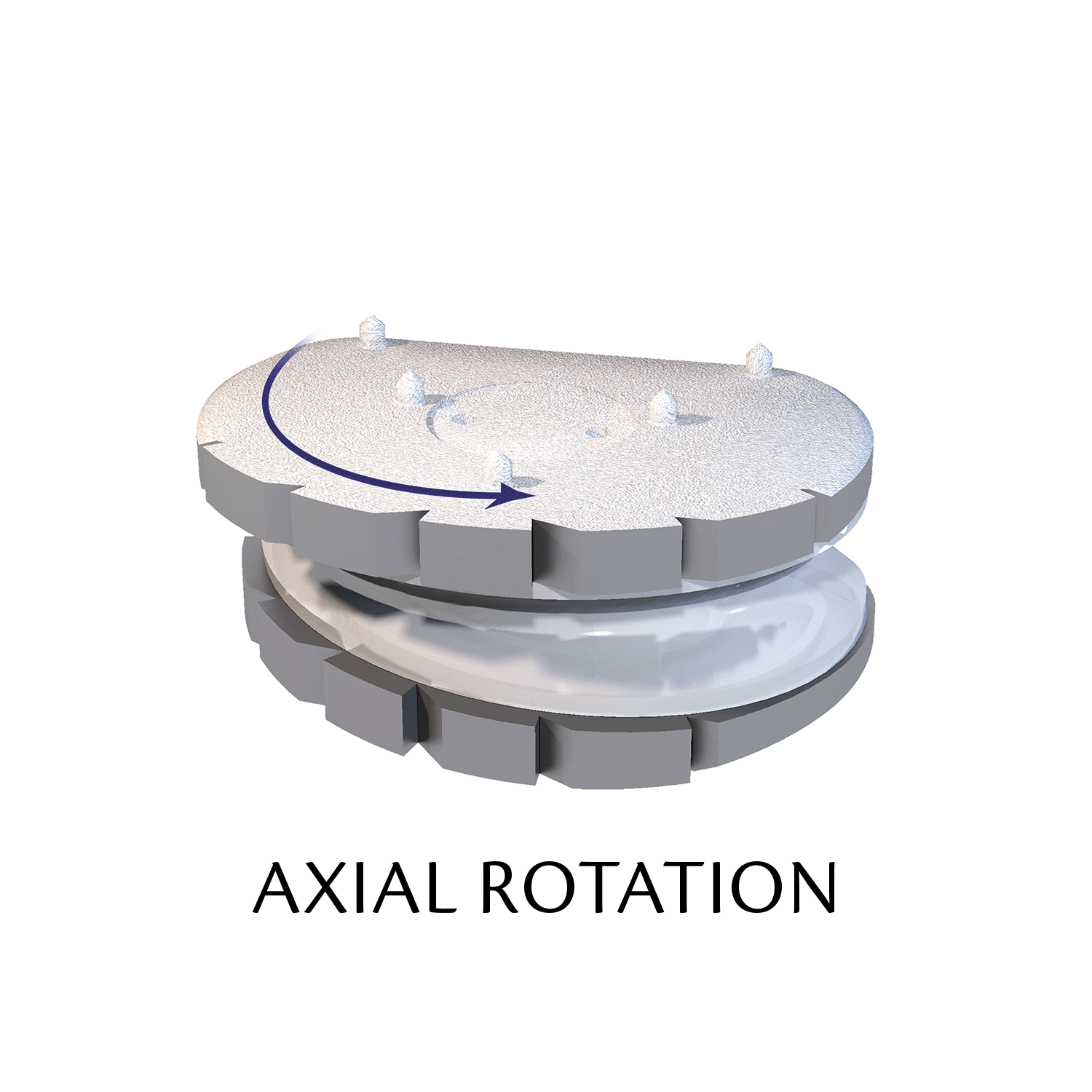
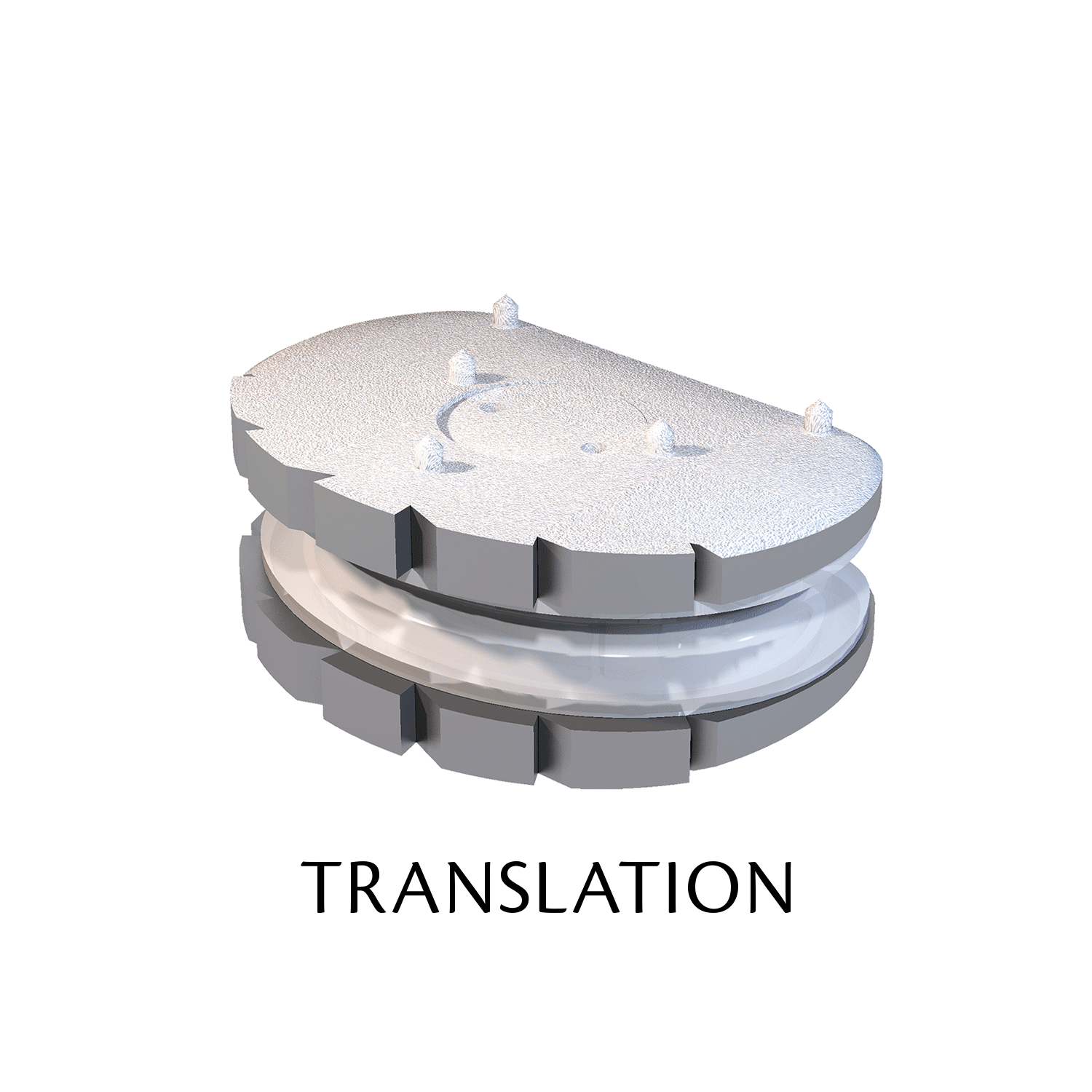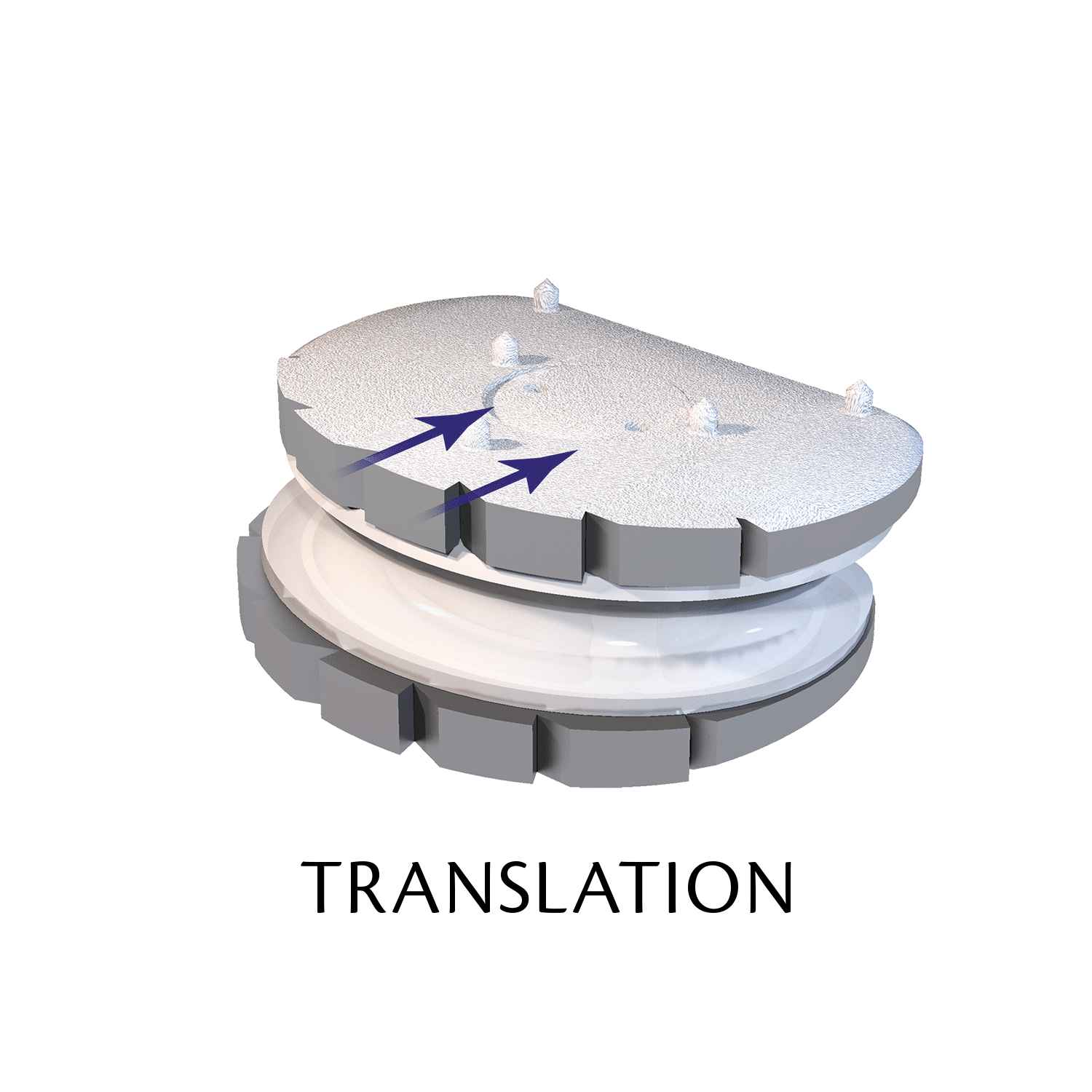
Lumbar prosthesis LP-ESP
The ESP prosthetic disc is the result of 10 years of collaborative research work at the Hôpital Pitié Salpêtrière in Paris, the French Atomic Energy Commission (CEA) and the Université Paris VI, and was driven by the Oséo Innovation contest in France.
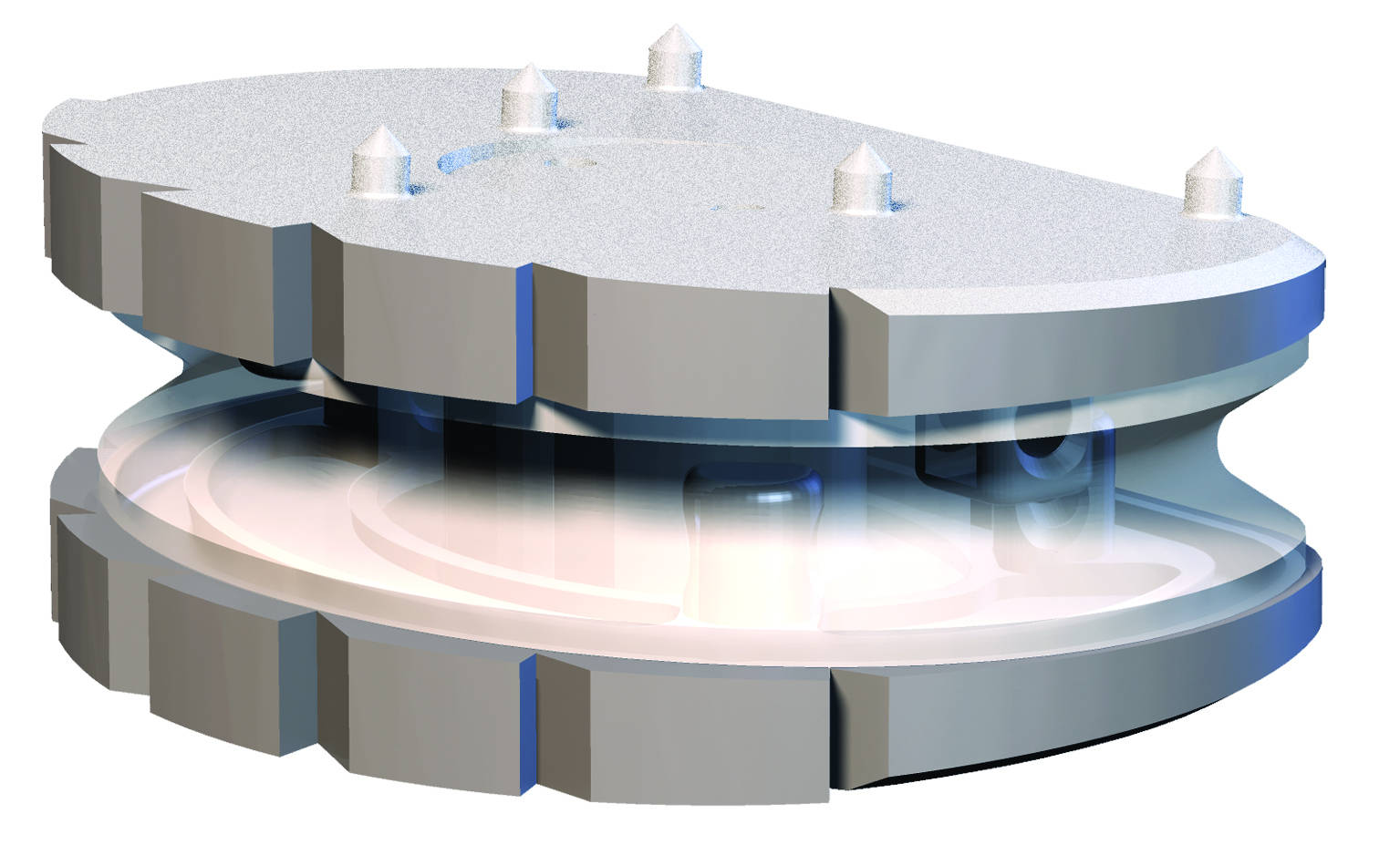
The LP-ESP lumbar disc prosthesis is designed for the treatment of degenerative disc disease (DDD) in the lumbar column. Disc condition is evaluated based on the convergence of data from clinical examinations and imaging.
The LP-ESP® lumbar disc prosthesis is a medical device for primary treatment.
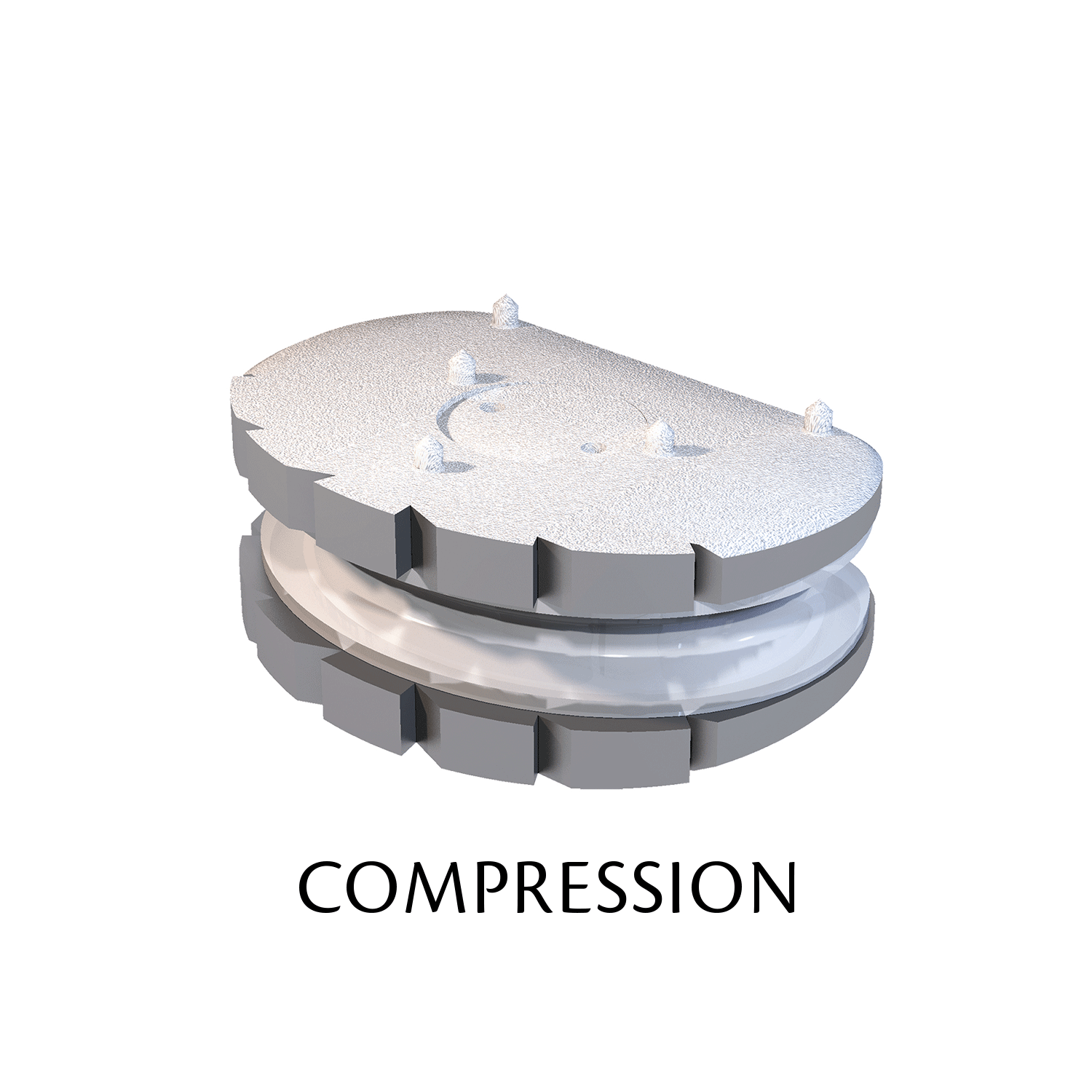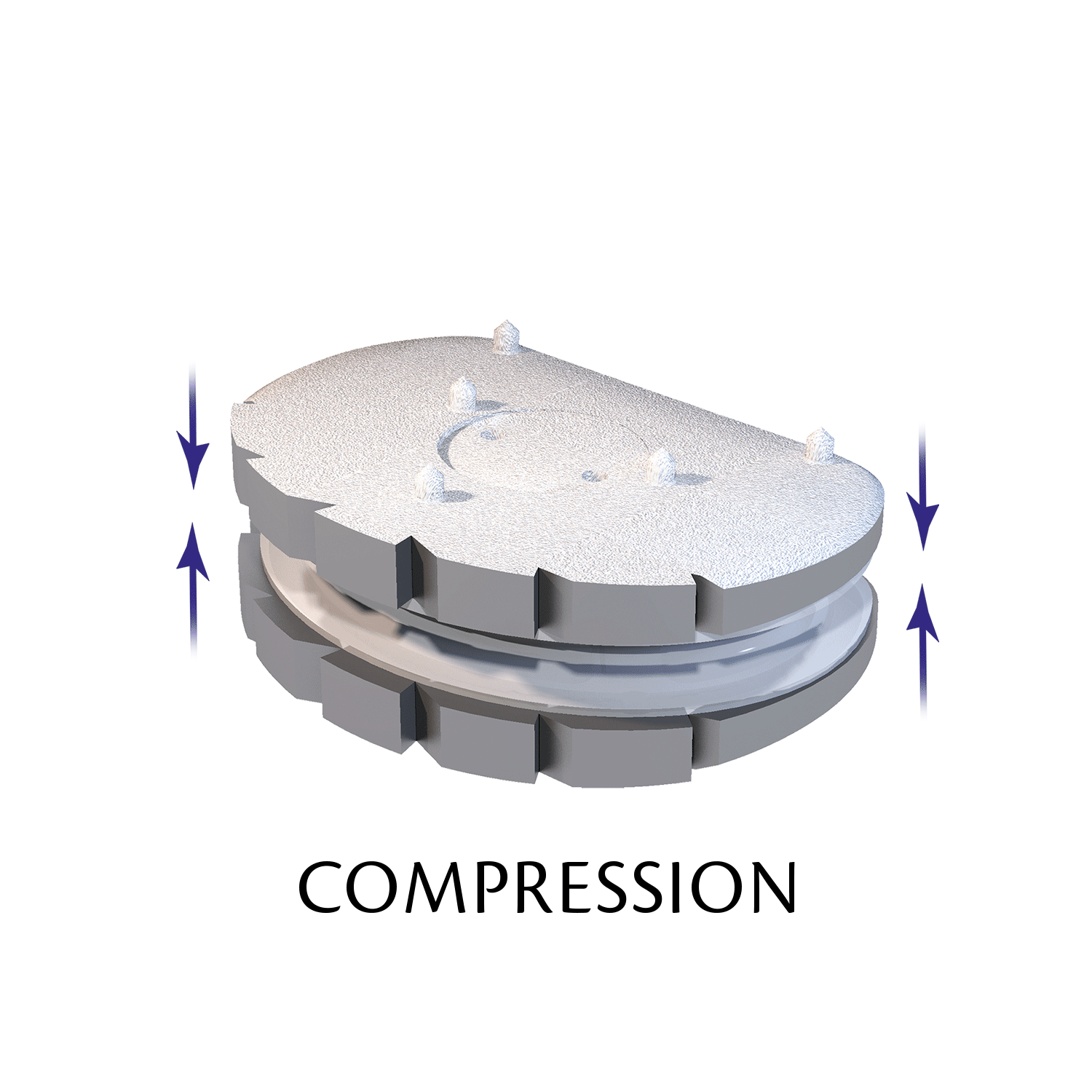
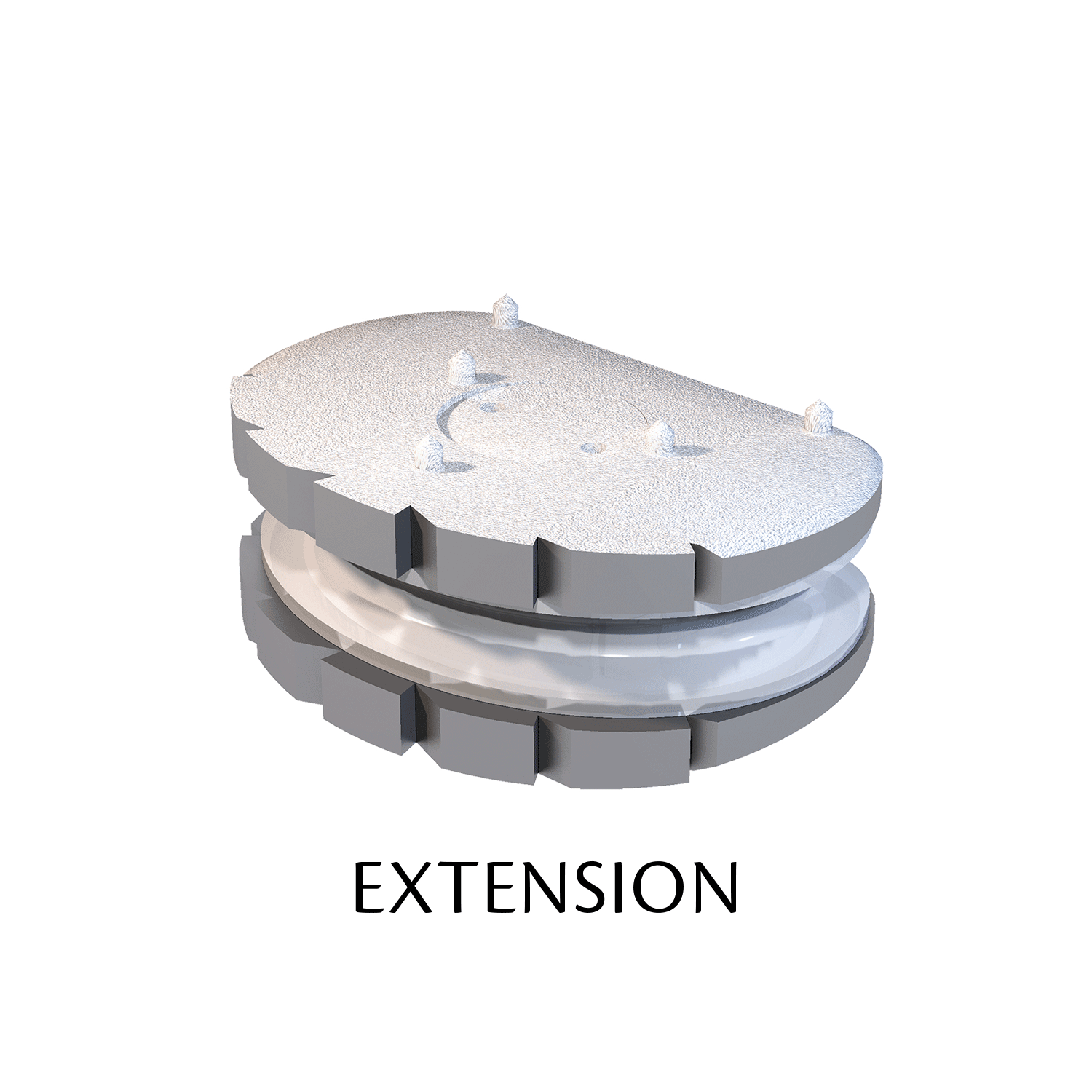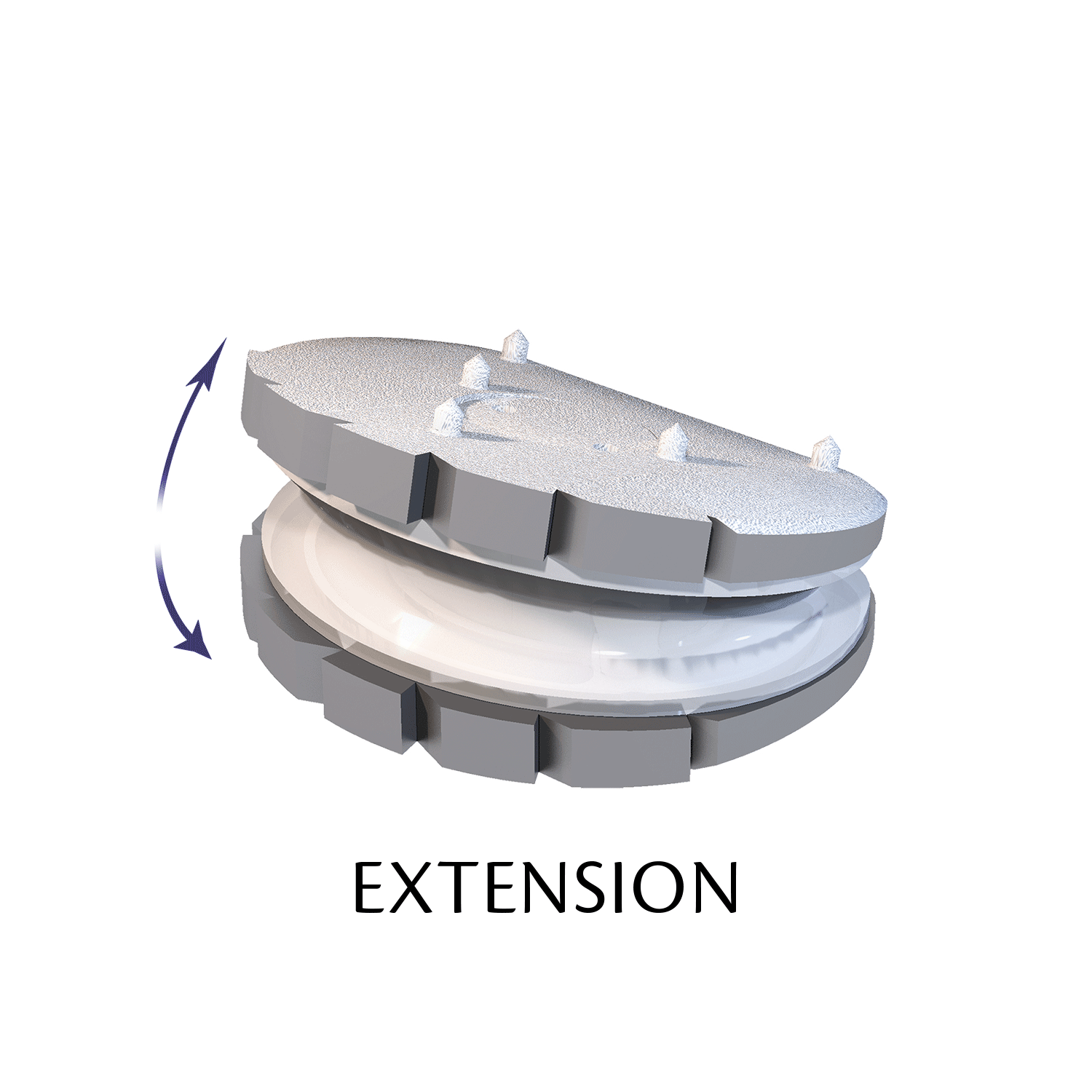
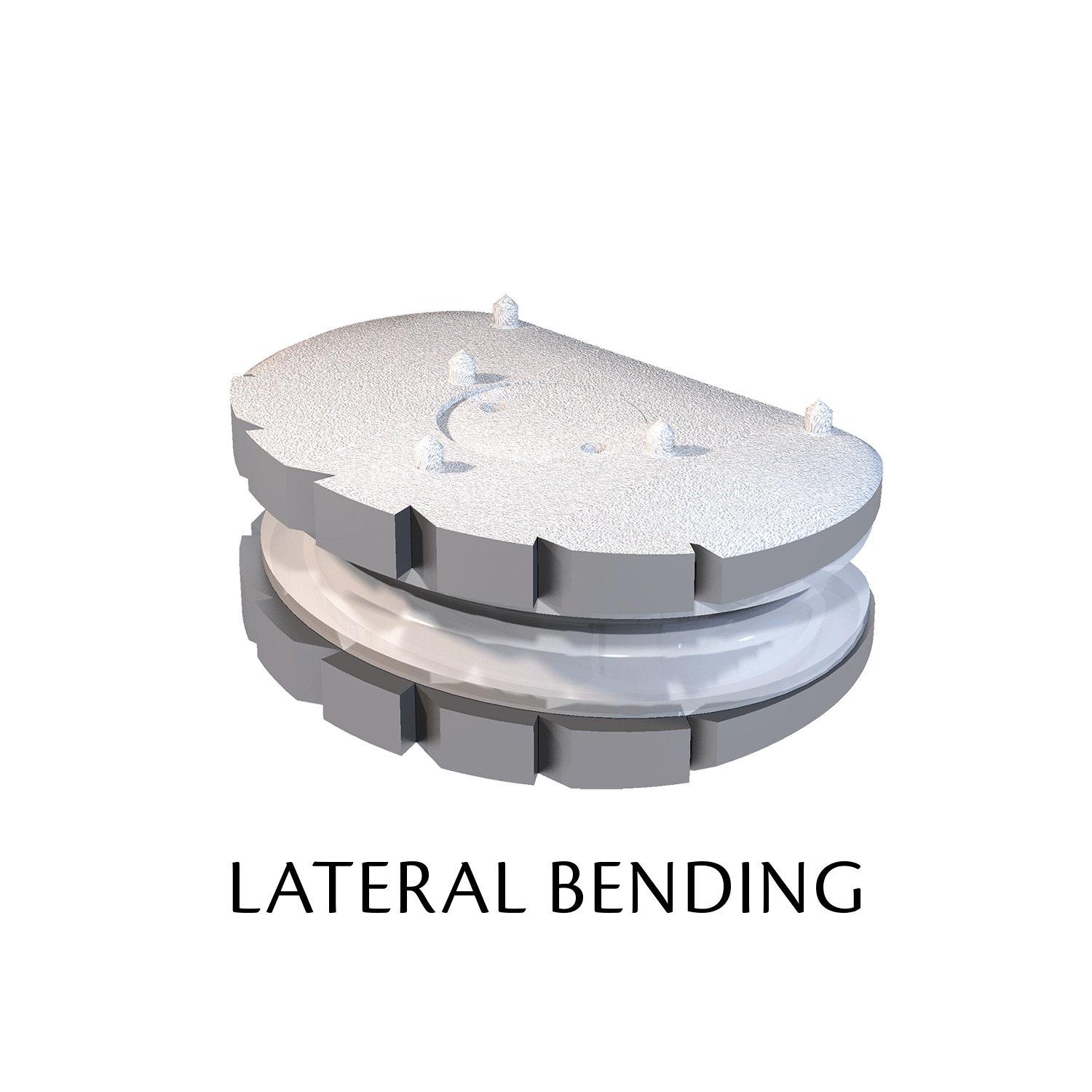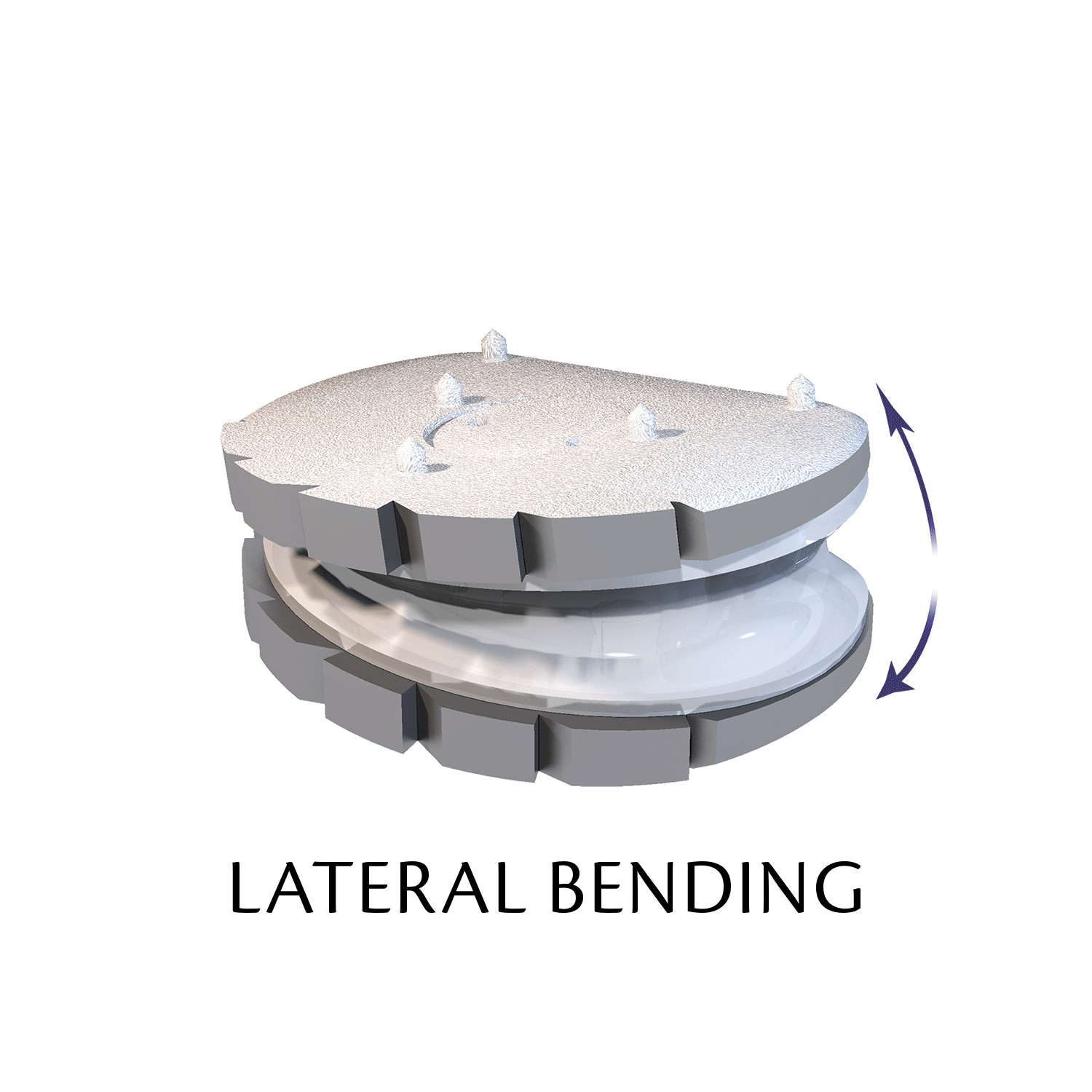
The ESP prosthetic disc restores the mechanical properties of the intervertebral disc and in particular its damping properties.
LP-ESP is CE marked and approved for use in Europe. It is not approved by FDA for use in the US.