VEOS
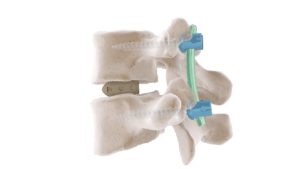
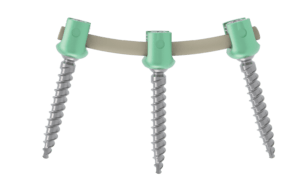
VEOS is a top loading posterior fixation system.
This device is a posterior osteosynthesis system. It is intended for the reduction of pathologies of the thoraco-lombo-sacral spine by restoring the disc height as well as the physiological bending of the spine.
Implants material: alliage de titane T6AV (ISO 5832-3 et ASTM F136).
Instruments material : stainless steel (EN 10088-2, EN 10216-5, ASTM A213), titanium (5832-3 or 5832-2), polymers
VEOS system can be used in skeletally mature individuals (adults) and in adolescent population for specific indications such as scoliosis.
This medical device is intended for the posterior fixation of the thoracic, lumbar and sacral spine.
It is indicated for the treatment of
- deformities (any etiology)
- Trauma
- Tumors
- The degenerative spine (spondylolisthesis, degenerative disc disease, spinal fractures, spinal stenosis, non-union).
VEOS can also be used in revision surgery in case of failure of a previous procedure.
VEOS is cleared by FDA for use in the US: K231658
OPEN THORACOLUMBAR ARTHRODESIS
VEOS PEEK
Flexible Posterior Osteosynthesis System
mont blanc 3D+
Coplanar Alignment System